Elida Betania Ariza Paez, Sergio Curcio, Natália Neme, Matheus Matos, Rodrigo S. Correa, Fabio Junio Pereira, Flaviane Francisco Hilário, Thiago Cazati, and Jason Guy Taylor. 2020. “
Synthesis, Photophysical and Electrochemical Properties of Novel and Highly Fluorescent Difluoroboron Flavanone β-Diketonate Complexes.” New J. Chem., Pp. -.
Publisher's VersionAbstractDifluoroboron β-diketonates complexes are highly luminescent with extensive properties such as their fluorescence both in solution and in solid state and their high molar extinction coefficients. Due to their rich optical properties, these compounds have been studied for their applications in organic electronics such as in self-assembly and applications in biosensors, bio-imaging and optoelectronic devices. The easy and fast synthesis of difluoroboron β-diketonate (BF2dbm) complexes makes their applications even more attractive. Although many different types of difluoroboron β-diketonates complexes have been studied, the cyclic flavanone analogues of these compounds have never been reported in the literature. Therefore, the present work aims to synthesize difluouroboron flavanone β-diketonate complexes, study their photophysical and electrochemical properties and assess their suitability for applications in optoelectronic devices. The synthesis was based on a Baker–Venkataraman reaction which initially provided substituted diketones, which were subsequently reacted with aldehydes to afford the proposed flavanones. The complexation was achieved by reacting flavanones and BF3. Et2O and in total 9 novel compounds were obtained. A representative difluoroboron flavanone complex was subjected to single crystal X-ray diffraction to unequivocally confirm the chemical structure. A stability study indicated only partial degradation of these compounds over a few days in a protic solvent at elevated temperatures. Photophysical studies revealed that the substituent groups and the solvent media significantly influence the electrochemical and photophysical properties of the final compounds, especially the molar absorption coefficient, fluorescence quantum yields, and the band gap. Moreover, the compounds exhibited a single excited-state lifetime in all studied solvent. Computational studies were employed to evaluate ground and excited states properties and carry out DFT and TDDFT level analysis. These studies clarify the role of each state in the experimental absorption spectra as well as the effect of the solvent.
Débora N. [de Freitas], Bruno H.S. Mendonça, Mateus H. Köhler, Marcia C. Barbosa, Matheus J. S. Matos, Ronaldo J. C. Batista, and Alan B. [de Oliveira]. 2020. “
Water Diffusion in Carbon Nanotubes Under Directional Electric Fields: Coupling Between Mobility and Hydrogen Bonding.” Chemical Physics, Pp. 110849.
Publisher's VersionAbstractMolecular Dynamics simulations of water confined in carbon nanotubes subjected to external electric fields show that water mobility strongly depends on the confining geometry, the intensity and directionality of the electric field. While fields forming angles of 0° and 45° slow down the water dynamics by increasing organization, perpendicular fields can enhance water diffusion by decreasing hydrogen bond formation. For 1.2 diameter long nanotubes, the parallel field destroys the ice-like water structure increasing mobility. These results indicate that the structure and dynamics of confined water are extremely sensitive to external fields and can be used to facilitate filtration processes.
Luiz G. Pimenta Martins, Diego L. Silva, Jesse S. Smith, Ang-Yu Lu, Cong Su, Marek Hempel, Connor Occhialini, Xiang Ji, Ricardo Pablo, Rafael S. Alencar, Alan C.R. Souza, Alysson A. Pinto, Alan B. de Oliveira, Ronaldo J. C. Batista, Tomás Palacios, Mário S. C. Mazzoni, Matheus J. S. Matos, Riccardo Comin, Jing Kong, and Luiz G. Cançado. 2020. “
Hard, transparent, sp3-containing 2D phase formed from few-layer graphene under compression.” Carbon.
Publisher's VersionAbstractDespite several theoretically proposed two-dimensional (2D) diamond structures, experimental efforts to obtain such structures are in initial stage. Recent high-pressure experiments provided significant advancements in the field, however, expected properties of a 2D-like diamond such as sp3 content, transparency and hardness, have not been observed together in a compressed graphene system. Here, we compress few-layer graphene samples on SiO2/Si substrate in water and provide experimental evidence for the formation of a quenchable hard, transparent, sp3-containing 2D phase. Our Raman spectroscopy data indicates phase transition and a surprisingly similar critical pressure for two-, five-layer graphene and graphite in the 4-6 GPa range, as evidenced by changes in several Raman features, combined with a lack of evidence of significant pressure gradients or local non-hydrostatic stress components of the pressure medium up to ≈ 8 GPa. The new phase is transparent and hard, as evidenced from indentation marks on the SiO2 substrate, a material considerably harder than graphene systems. Furthermore, we report the lowest critical pressure (≈ 4 GPa) in graphite, which we attribute to the role of water in facilitating the phase transition. Theoretical calculations and experimental data indicate a novel, surface-to-bulk phase transition mechanism that gives hint of diamondene formation.
Ronaldo J. C. Batista, Rafael F. Dias, Ana P. M. Barboza, Alan B. de Oliveira, Taise M. Manhabosco, Thiago R. Gomes-Silva, Andreij C. Gadellha, Cassiano Rabelo, Luiz G. L. Cançado, Ado Jorio, Hélio Chacham, and Bernardo R. A. Neves. 2020. “
Nanomechanics of few-layer materials: do individual layers slide upon folding?.” Beilstein J. Nanotechnol., 11, Pp. 1801–1808.
Marcelo Fernandes Cipreste, Wagner [da Nova Mussel], Juliana [Batista da Silva], Maria [Betânia Freitas de Marques], Ronaldo Junio [Campos Batista], Pedro Lana Gastelois, Waldemar [Augusto Almeida de Macedo], and Edésia Martins [Barros de Sousa]. 2020. “
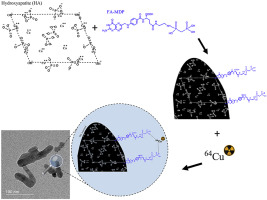
A new theranostic system for bone disorders: Functionalized folate-MDP hydroxyapatite nanoparticles with radiolabeled copper-64.” Materials Chemistry and Physics, Pp. 123265.
Publisher's VersionAbstractHydroxyapatite nanoparticles have been investigated as biological agents for the treatment and diagnosis of bone diseases due to their properties, providing high affinity to bone tissues and also due to the possibility to chemically modify the surfaces of these nanoparticles to provide active targeting to bone tumors or other bone disorders. In this work, synthetic hydroxyapatite nanoparticles and their surface modifications with folic and medronic acid were studied. Copper-64 was produced by neutron irradiation in a TRIGA MARK I nuclear reactor, and the functionalized nanoparticles radiolabeled with this radioisotope. The multi-technique characterization includes FTIR, PXRD, TGA, DSC, CHN, Zeta potential, XPS, SEM, TEM, and Gamma spectroscopy. Furthermore, the evaluation of the chemical interaction stability was through leaching tested for efficiency. The results indicate that folic and medronic acids can be covalently bonded to HA surface, producing a new material not yet described in the literature, been stably attached to hydroxyapatite nanoparticle surfaces, able to provide active targeting for bone disorders. The complexation of copper-64 provides high radiochemistry purity, although the specific activity must be improved.